Is Sr Oh 2 Soluble in Water
Is SrOH2 soluble in water. Using solubility guidelines predict whether each of the following compounds is soluble or insoluble in water.

Chemsheets As006 Electron Arrangement Ppt Download
O2- OH-Insoluble except alkali metals and NH 4 Ca 2 Sr 2 Ba 2 somewhat soluble CO 3 2- PO 4 2- S 2- SO 3 2- C 2O4 2- CrO 4 2-Insoluble except alkali metals and NH 4 Example 1.

. NaCl is soluble will completely dissolve because Na is an alkali metal and is. Because SrOH 2 is slightly soluble in cold water its preparation can be easily carried out by the addition of a strong base such as NaOH or KOH drop by drop to a solution of any soluble strontium salt most commonly SrNO 3 2 strontium nitrate. This problem has been solved.
It is an ionic compound which readily dis. The answer Strontium nitrate is soluble in water. The SrOH 2 will precipitate out as a fine white powder.
Because SrOH 2 is only slightly soluble in cold water it is simple to prepare by adding a strong base such as NaOH or KOH drop by drop to a solution of any soluble strontium salt most commonly SrNO 3 2 strontium nitrate. Strontium hydroxide Sr OH2 is not very soluble in water. NaOH will dissolve to the extent of 1110 gL at 20º C KOH is pretty similar at 1210 gL at 25º C.
From here the solution is filtered and the SrOH 2 is. Assuming this information to be accurate it is safe to conclude that 0400 g would dissolve in 2500. All alkali metals hydroxides are soluble in water.
Helmenstine Anne Marie PhD. Sr s 2H 2 O g - SrOH 2 aq H 2 g Solubility of strontium and strontium compounds. The answer Strontium hydroxide is soluble in water.
2 is more or less soluble in water than is MgOH 2. Which of the following compounds is least soluble in water. The solution is then filtered and the SrOH 2 is washed and dried with cold water.
Strontium compounds can be water soluble. Now for the other two. Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent.
Trend in solubility of group 2 sulfates-decreases down group as contain doubly charged -ions MgSO4 soluble. The SrOH 2 will precipitate out as a fine white powder. What is Soluble and Insoluble.
Ca OH 2 is slightly soluble. All sulfides are insoluble except those of the Group 1 and Group 2 elements and NH 4. Geetha Meritnation Expert added an answer on 311016.
Alkali earth metals Only strontium hydroxide SrOH 2 and barium hydroxides BaOH 2 are completely soluble from alkaline earth metals. The Ksp of CaOH2 is 55 x 10-6 and the Ksp of MgOH2 is 561 x 10-12. All hydroxides are insoluble except those of the Group 1 elements Ba OH 2 and Sr OH 2.
So the increasing order is. SrCl 2 SrSO 4 Sr 3 PO 4 2 SrS Sr OH 2 SrCO 3 Sr NO 3 2. Fizzes in water and forms colourless solution.
It is easy to state which ones are most certainly very water soluble. Suggest whether SrOH 2 would be more or less soluble in hot. Higher the hydration energy compared to lattice energy higher will be the solubility.
SrOH2 soluble BaOH2 soluble. State any assumptions you make. ZnSO4 SrOH2 MgBr2 PbI2 NH42CO3 Question.
If an aqueous solution of ____ is added to a mixture of Pb2 and Ba2 the lead ion will precipitate but the barium ion will remain in. Examples include strontium carbonate with a water solubility of 10 mgL and strontium chromate with a water solubility of 9 mgL. Neither of them is very soluble.
Want this question answered. This is due to increase in size of the cation and decrease in their lattice energies on moving down the group. From here the solution is filtered and the SrOH 2 is washed with cold.
SrOH will form a fine white powder as it precipitates. Is SrOH2 Strontium hydroxide soluble or insoluble in water. It is an ionic compound which readily dissoc.
Sr observations w water. How many of the following are soluble in water. Sr OH2 Strontium hydroxide is Soluble in water.
Hydration energies become more due to decrease in lattice energies and so the solubility of hydroxide in water. 2 rows It can be prepared by the reaction of water upon the oxide but because SrOH 2 is very. Strontium is water insoluble but it does react with water.
Because SrOH 2 is slightly soluble in cold water its preparation can be easily carried out by the addition of a strong base such as NaOH or KOH drop by drop to a solution of any soluble strontium salt most commonly SrNO 3 2 strontium nitrate. Both NaOH and KOH are highly water soluble. Fizzes in water and after while solid forms CaOH2 which is slightly soluble in water pH 11-13.
Be OH 2 and Mg OH 2 are almost insoluble Ca OH 2 is sparingly soluble while Sr OH 2 and Ba OH 2 are fairly soluble. Solubility of metallic hydroxides increases down the group in the alkaline earth metal. Is SrNO32 Strontium nitrate soluble or insoluble in water.
2-Soluble except Sr 2 Ba 2 Ca 2 Pb 2 Generally insoluble will not dissociate in water unless bonded to. Mg OH 2 Ca OH 2 Sr OH 2 Ba OH 2. Be notified when an answer is posted.

How To Write The Net Ionic Equation For Na2so4 Sr No3 2 Nano3 Srso4 Youtube
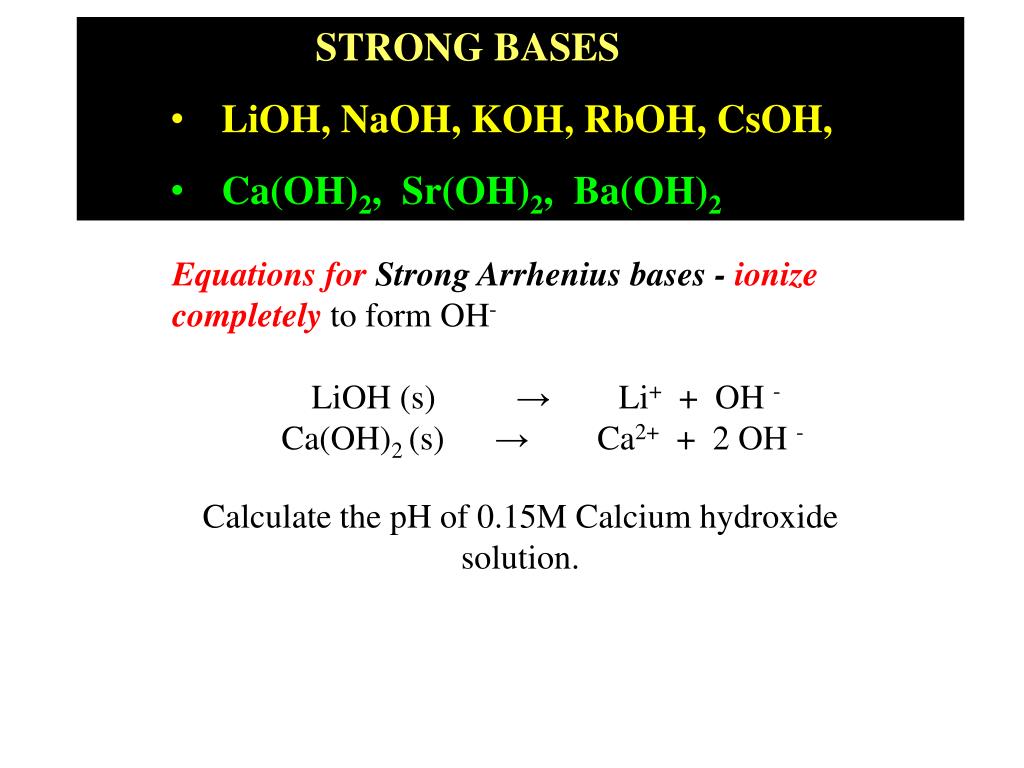
Ppt Strong Bases Lioh Naoh Koh Rboh Csoh Ca Oh 2 Sr Oh 2 Ba Oh 2 Powerpoint Presentation Id 5872807
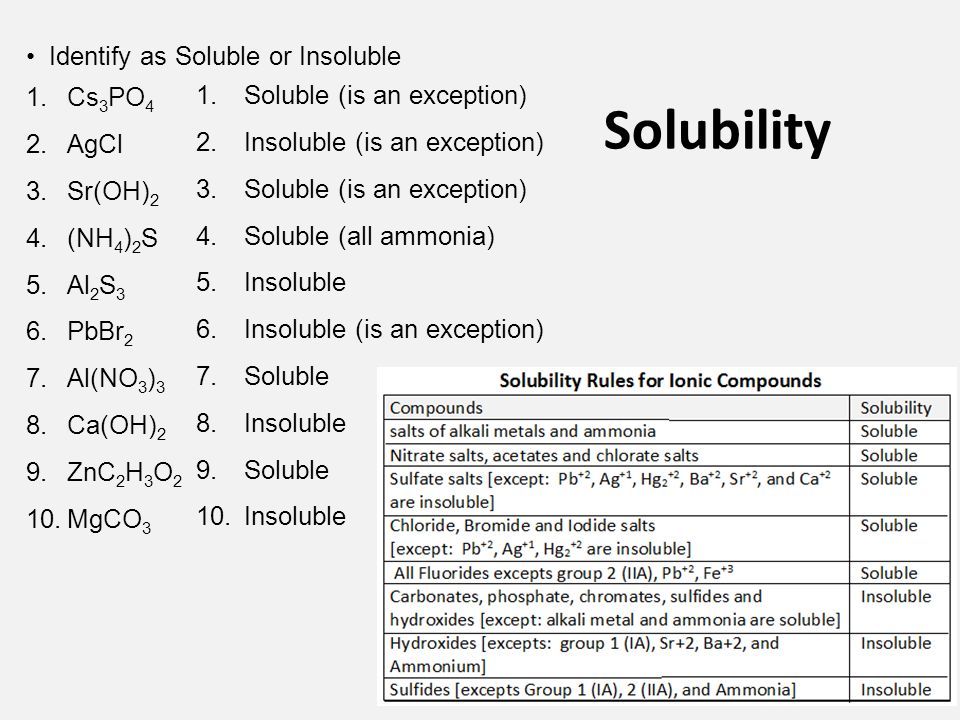
Solutions Solubility Terminology A Solution Is A Mixture In Which Particles Of One Or More Substances The Solute Are Distributed Uniformly Throughout Ppt Download

Is Sr Oh 2 Soluble Or Insoluble In Water Youtube

Group 2 Elements Compounds Ppt Video Online Download

How To Write The Net Ionic Equation For Sr Oh 2 H3po4 Sr3 Po4 2 H2o Youtube

Arrange The Following In The Order Of Property Mentioned Be Oh 2 Mg Oh 2 Sr Oh 2 Ba Oh 2 Decreasing Solubility In Water

Selected Solubility Product Constants Compound Formula K Sp Download Table

Is Sr Oh 2 Strontium Hydroxide An Acid Base Or Neutral Youtube

Is Sr Oh 2 Soluble Or Insoluble In Water Youtube

Equation For Sr Oh 2 H2o Strontium Hydroxide Water Youtube

Equation For Sr Oh 2 H2o Strontium Hydroxide Water Youtube
How To Write The Net Ionic Equation For Hclo4 Sr Oh 2 Sr Clo4 2 H2o Youtube

Chemsheets As006 Electron Arrangement Ppt Download

Chapter 4 Solution Chemistry And The Hydrosphere Ppt Video Online Download

How To Write The Net Ionic Equation For Sr Oh 2 H3po4 Sr3 Po4 2 H2o Youtube
How To Write The Net Ionic Equation For Hcl Sr Oh 2 H2o Srcl2 Youtube

Pdf Nanoparticles Of Sr Oh 2 Synthesis In Homogeneous Phase At Low Temperature And Application For Cultural Heritage Artefacts

Comments
Post a Comment